
Scandium, symbolized by ‘Sc’ with an atomic number of 21, is a rare and unique metal. Lars Fredrik Nilson discovered it in 1879 in Scandinavia, and the element was named after the region. Although scandium is the 50th most abundant element on Earth, it occurs in trace amounts in over 800 minerals. This scarcity makes it a rare and expensive resource. Despite these challenges, scandium has found important uses in various industries due to its special properties, such as a high melting point, low density, and ability to form strong alloys.
Scandium in the Aerospace Industry
Scandium is valuable in the aerospace industry, especially in forming strong, lightweight alloys with aluminum. These aluminum-scandium alloys are essential for making aircraft components. They improve high-strength aluminum by reducing grain growth at high temperatures, making aerospace parts more durable. Scandium’s role is particularly important in military aircraft, where strength and weight are critical. However, its high cost limits its widespread use.

Scandium in Lighting Technology
Scandium also improves modern lighting. Scandium iodide is used in metal halide lamps, a type of mercury vapor lamp that produces light similar to natural sunlight. This light is essential in the film and television industries, where accurate color reproduction matters. Scandium oxide (Sc2O3), also known as scandia, is used in high-intensity lamps, such as those found in stadiums and large public spaces. These applications make use of scandium’s ability to enhance light intensity and quality.
Scandium in Sporting Goods
Scandium alloys have transformed the design of sports equipment. They are used in making baseball bats, lacrosse sticks, bicycle frames, and golf iron shafts. Scandium improves the strength of aluminum while keeping it lightweight. This combination results in durable and easy-to-handle sports equipment, giving athletes an advantage.

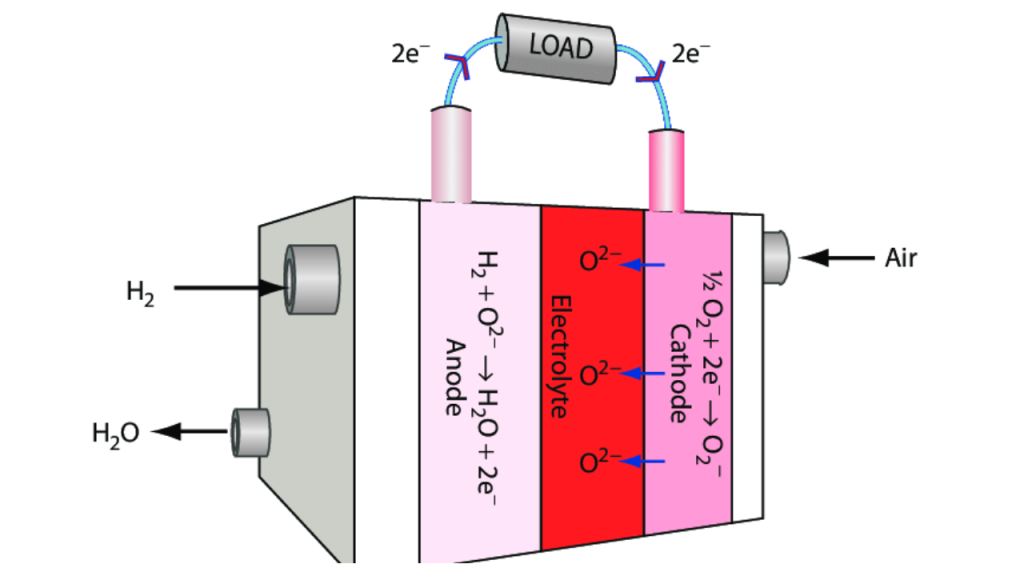
Scandium in Solid Oxide Fuel Cells (SOFCs)
Scandium is also important in clean energy technology. It is used in solid oxide fuel cells (SOFCs), which convert chemical energy into electricity without combustion. Scandium oxide is the electrolyte material in these cells, improving their efficiency by lowering the operating temperature and increasing ionic conductivity. This application is vital as the world shifts toward more sustainable energy sources.
Scandium in Oil Refining and Agriculture
Scandium’s usefulness extends to oil refining. The radioactive isotope scandium-46 is used as a tracer in refining processes. It helps monitor the movement of different fractions and can detect leaks in underground pipelines. In agriculture, scandium sulfate is used in small amounts to improve the germination of seeds like corn, peas, and wheat.
Conclusion
Scandium is a rare and valuable element with many uses across different industries. From aerospace and sports equipment to lighting and energy, scandium’s properties make it essential in modern technology. As research continues, scandium’s role in advanced materials and technologies is likely to grow, making it even more important in the future.
Read more: Scandium Introduction: Structure, Classification, and Characteristics
