
Introduction
In the expansive landscape of catalysis, the discovery and application of novel compounds can significantly influence the progress of chemical synthesis. Among the array of catalysts, scandium triflate (Sc(OTf)3) has emerged as a fascinating and versatile compound, captivating the attention of chemists worldwide. With its distinctive properties and catalytic prowess, scandium triflate has found applications in diverse fields, ranging from organic synthesis to polymerization reactions.
Chemical Structure and Properties
Scandium triflate unveils its catalytic prowess through a meticulously crafted chemical structure. This coordination compound seamlessly integrates scandium, a transition metal renowned for its unique electronic properties, with trifluoromethanesulfonate (OTf) ligands. This marriage of elements gives rise to a molecular architecture that transcends its components, transforming into a potent Lewis acid catalyst.
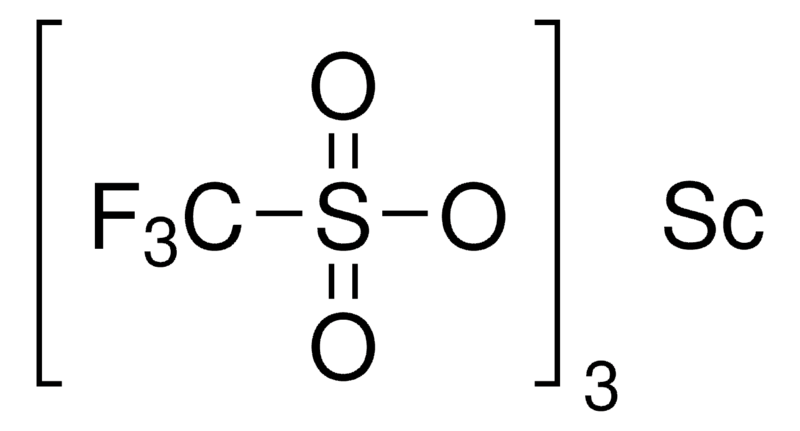
The triflate ligands, with their electron-withdrawing nature, play a pivotal role in shaping the reactivity of scandium triflate. By fostering a strong Lewis acidity, they bestow upon the compound the ability to initiate and catalyze a diverse array of chemical reactions. This inherent property makes scandium triflate a stalwart companion in the intricacies of organic transformations.
Beyond its catalytic finesse, scandium triflate stands firm in the face of thermal challenges. Exhibiting high thermal stability, this compound fearlessly navigates a wide range of reaction conditions. This attribute not only enhances its reliability in various chemical processes but also broadens its application spectrum.
Moreover, the solubility profile of scandium triflate adds another layer of versatility to its repertoire. Dissolving seamlessly in various organic solvents, the compound seamlessly integrates into diverse reaction media. This solubility extends its reach across different chemical environments, making it an adaptable catalyst for reactions with varying polarities and requirements.
In essence, the chemical structure and properties of scandium triflate intertwine to create a catalyst that is not only powerful in its reactivity but also resilient in the face of diverse reaction challenges. The marriage of scandium and triflate emerges as a harmonious symphony, conducting a melody of catalysis that resonates across a broad spectrum of chemical transformations.
Catalytic Applications
Organic Synthesis:
Scandium triflate has gained prominence as a catalyst in organic synthesis due to its ability to activate a variety of substrates. Its Lewis acid character facilitates the generation of carbocations, allowing for the initiation of key reactions such as Friedel-Crafts alkylation and acylation. The use of scandium triflate in these reactions often leads to improved selectivity and higher yields compared to traditional catalysts.
One notable example is its application in the synthesis of biologically active compounds. Scandium triflate has proven effective in promoting reactions that lead to the formation of complex structures, crucial in medicinal chemistry. Researchers have utilized its catalytic power to streamline synthetic routes, reducing the number of steps and increasing overall efficiency.
Olefin Polymerization:
The catalytic potential of scandium triflate extends to the realm of olefin polymerization. Its use as a catalyst in polymerization reactions has demonstrated exceptional control over polymer chain length and molecular weight. This control is crucial in the synthesis of well-defined polymers with tailored properties, essential for the development of advanced materials.
Scandium triflate has shown promise in the polymerization of ethylene and propylene, producing polymers with unique structures and properties. This versatility opens avenues for the design of polymers with specific applications, from advanced packaging materials to high-performance engineering plastics.
Asymmetric Catalysis:
Scandium triflate has also found application in asymmetric catalysis, a field of immense importance in the synthesis of pharmaceuticals and fine chemicals. Its ability to induce enantioselectivity in certain reactions makes it a key player in the development of efficient and selective synthetic routes.
In the realm of drug synthesis, scandium triflate has demonstrated its utility in creating chiral building blocks. The control it provides over the stereochemistry of reactions is crucial for producing pharmaceutical compounds with desired biological activities.
Catalytic Rearrangements:
The Lewis acidity of scandium triflate facilitates catalytic rearrangements, enabling the conversion of one isomer into another. This property is particularly valuable in the synthesis of complex organic molecules where regio- and stereochemical control are paramount.
Applications in the synthesis of natural products and bioactive compounds showcase the utility of scandium triflate in orchestrating intricate rearrangement reactions. The ability to precisely control molecular transformations enhances the efficiency of these syntheses, offering new routes to compounds with therapeutic potential.

Environmental and Economic Considerations
The catalytic efficiency of scandium triflate has led to increased interest in its use as an environmentally benign alternative to traditional catalysts. Its ability to promote reactions under mild conditions reduces energy consumption and minimizes the generation of by-products, aligning with the principles of green chemistry.
Furthermore, scandium triflate’s catalytic prowess often translates into higher reaction yields, reducing the overall amount of reactants needed. This not only contributes to cost-effectiveness but also aligns with the broader goal of sustainable chemical synthesis.
Challenges and Future Prospects
Despite its many advantages, the utilization of scandium triflate is not without challenges. The scarcity and high cost of scandium present economic hurdles for widespread adoption. Researchers are actively exploring ways to mitigate these challenges, such as developing more efficient recycling methods to minimize the need for large quantities of scandium triflate in reactions.
Looking forward, the future of scandium triflate in catalysis appears promising. Ongoing research efforts focus on expanding its catalytic repertoire, discovering novel applications, and improving its overall sustainability. Collaboration between academia and industry is essential to overcoming challenges and unlocking the full potential of scandium triflate in various chemical processes.
Research endeavors are also directed towards understanding the fundamental mechanisms of scandium triflate catalysis. A deeper insight into its reactivity and selectivity will likely lead to the development of more sophisticated catalysts inspired by the unique properties of scandium triflate.
Conclusion
In the dynamic landscape of catalysis, scandium triflate stands out as a catalyst with unparalleled versatility. Its unique combination of Lewis acidity, thermal stability, and solubility in organic solvents has positioned it as a valuable tool in the hands of researchers and industrial chemists alike. From driving organic synthesis to influencing polymerization reactions and catalyzing asymmetric transformations, scandium triflate continues to shape the landscape of modern chemistry.
For more information about scandium triflate or other scandium materials, please visit our homepage.

I’m not that much of a internet reader to be honest but your sites really nice, keep it up! I’ll go ahead and bookmark your site to come back down the road. All the best
Wow that was unusual. I just wrote an extremely long comment but after I clicked submit my comment didn’t appear. Grrrr… well I’m not writing all that over again. Anyways, just wanted to say excellent blog!
Sweet blog! I found it while surfing around on Yahoo News. Do you have any suggestions on how to get listed in Yahoo News? I’ve been trying for a while but I never seem to get there! Thanks
Greetings from Los angeles! I’m bored at work so I decided to browse your site on my iphone during lunch break. I love the knowledge you present here and can’t wait to take a look when I get home. I’m surprised at how fast your blog loaded on my cell phone .. I’m not even using WIFI, just 3G .. Anyways, very good blog!