
Introduction
Scandium nitrate is important in materials science and catalysis, playing a unique and crucial role among chemical compounds. Composed of scandium, nitrogen, and oxygen (Sc(NO₃)₃), this compound serves as a gateway to exploring scandium’s unique properties and its integration into cutting-edge technologies. Its relevance stretches across various sectors, from electronics to energy, offering solutions and enhancements to longstanding challenges.
Chemical and Physical Properties
Scandium nitrate is characterized by its crystalline form and high solubility in water, attributes that lend it well to a range of applications. At the molecular level, the scandium ion is trivalent, meaning it carries a charge of +3, which forms ionic bonds with three nitrate anions, each carrying a charge of -1. This ionic structure contributes to its solubility and reactivity, making it a versatile reagent in chemical synthesis.
Physically, scandium nitrate appears as a white to off-white crystalline powder. It decomposes upon heating, yielding scandium oxide and nitrogen oxides, indicating its thermal sensitivity. This decomposition behavior is crucial in applications requiring controlled thermal processing, such as in the synthesis of scandium-doped ceramics and glasses.
Production and Synthesis
Synthesizing scandium nitrate involves the reaction of either scandium oxide (Sc2O3) or scandium metal with nitric acid (HNO3). The process typically yields scandium nitrate in aqueous solution, which can then be crystallized.
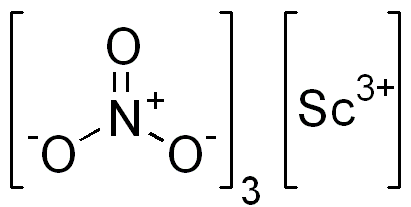
The production of scandium nitrate, and scandium compounds in general, is hampered by the scarcity of scandium itself. Scandium is not found in large concentrations in any specific location, making its extraction and purification both challenging and expensive. Advances in extraction technology and the recycling of scandium from industrial waste have become increasingly important to meet the growing demand.
Applications
Scandium nitrate’s applications leverage its unique chemical properties to improve the performance and functionality of a wide range of materials and processes. Its versatility can be attributed to scandium’s ability to alter the electronic and structural properties of materials, enhancing their performance in various applications.
Electronics and Optoelectronics
In the realm of electronics, scandium nitrate plays a crucial role in the development of high-efficiency lighting and display technologies. When used as a dopant in solid-state lighting devices, such as light-emitting diodes (LEDs), scandium nitrate can significantly enhance the light output and color rendering of these devices. This improvement is due to scandium’s ability to modify the electronic structure of the host material, leading to more efficient energy conversion and improved luminous efficiency.
Furthermore, scandium-doped materials, synthesized using scandium nitrate, are being explored for their potential in optoelectronic applications. These materials can offer superior performance in lasers, photodetectors, and solar cells, where the precise control of electronic properties is crucial for efficiency and reliability.
Catalysts in Chemical Synthesis
Scandium nitrate’s role as a catalyst is particularly notable in organic synthesis, where it facilitates a variety of chemical reactions, including polymerization, esterification, and the synthesis of cyclic compounds. Its effectiveness as a catalyst stems from scandium’s ability to form stable, yet reactive, intermediates that can lower the energy barrier for reaction pathways, leading to higher yields and selectivity. This catalytic activity is invaluable in the pharmaceutical industry, where the synthesis of complex molecules requires precision and efficiency.
Advanced Ceramics and Glass
The addition of scandium nitrate to ceramics and glass materials can result in significant improvements in their mechanical and thermal properties. In ceramics, scandium acts as a grain refiner, enhancing the material’s strength and resistance to thermal shock. This is crucial for applications in high-temperature environments, such as in aerospace and automotive components, where materials must withstand extreme conditions without failure.
In glass manufacturing, scandium nitrate contributes to the development of glasses with improved optical properties, such as increased refractive index and resistance to thermal expansion. These properties are essential for high-performance optical components, including lenses, mirrors, and fiber optics, where dimensional stability and optical clarity are paramount.
Research and Emerging Technologies
The potential applications of scandium nitrate extend into cutting-edge research and emerging technologies. One area of exploration is in energy storage, where scandium-doped electrodes are being investigated for use in lithium-ion batteries and fuel cells. The addition of scandium can improve the electrical conductivity and stability of the electrode materials, leading to batteries with higher energy densities and longer lifespans.
Another promising area is in the field of additive manufacturing (3D printing), where scandium nitrate can be used to synthesize scandium-alloy powders. These powders can produce components with superior strength and lighter weight, ideal for aerospace and automotive applications where material efficiency is key.
Environmental Remediation
Beyond its industrial applications, scandium nitrate is also being studied for its potential in environmental remediation. Its ability to catalyze the breakdown of pollutants, such as nitrogen oxides (NOx) and volatile organic compounds (VOCs), makes it a candidate for use in air purification systems and wastewater treatment processes. This catalytic activity could play a significant role in reducing the environmental impact of industrial processes and urban pollution.

Safety and Handling
Handling scandium nitrate necessitates caution due to its chemical reactivity and potential health hazards. Direct contact can cause skin and eye irritation, and its decomposition products are hazardous. Therefore, appropriate safety measures, including personal protective equipment (PPE) and proper ventilation, are essential. Environmental considerations also dictate careful disposal and recycling practices to prevent contamination of water bodies.
Market and Economic Aspects
The market for scandium nitrate is intricately linked to the availability of scandium and the demand for scandium-enhanced products. Its price is influenced by the limited scandium resources and the cost of extraction and purification. Despite these challenges, the growing interest in scandium’s applications has spurred investments in mining and recovery technologies, potentially easing supply constraints and lowering prices in the long term.
Conclusion
Scandium nitrate exemplifies the intersection of chemistry, materials science, and technological innovation. Its unique properties and applications underscore the ongoing need for research and development in understanding and utilizing scandium compounds. As technology advances, the potential for scandium nitrate to contribute to new materials and processes only expands, promising a future where its impact extends even further into our technological and material landscapes.
For more information about scandium triflate or other scandium materials, please visit our homepage.
