Introduction
Scandium (Sc) is a chemical element with the atomic number 21. It belongs to the transition metals group on the periodic table. Scandium was discovered in 1879 by a Swedish chemist named Lars Fredrik Nilson. He named it after Scandinavia, where the element was first identified. Scandium is rare and valuable. It is used to make strong alloys and special types of lighting. To understand why scandium is important, we need to look at its electron configuration.
Basics of Electron Configuration
Electron configuration shows how electrons are arranged in an atom. Electrons fill orbitals in a specific order. They start with the lowest energy level. This pattern is known as the Aufbau principle. By following this principle, we can determine the electron configuration for any element.
Scandium has 21 electrons. So, we start filling from the lowest energy levels. The electrons fill the orbitals in a sequence based on their energy.
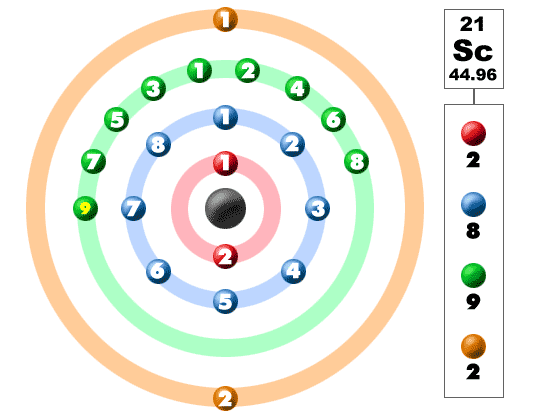
Scandium’s Electron Configuration
The electron configuration for scandium is ArArAr 3d¹ 4s². Here’s what this means:
- ArArAr: This represents the electron configuration of argon, the noble gas before scandium on the periodic table. Argon’s configuration is 1s² 2s² 2p⁶ 3s² 3p⁶. We use ArArAr to simplify scandium’s configuration and focus on the electrons that come after argon.
- 3d¹: After argon, scandium has one electron in the 3d orbital. This is the start of the d-block elements, where the d orbitals begin to fill.
- 4s²: Scandium also has two electrons in the 4s orbital. The 4s orbital is filled before the 3d, even though it is written after in the notation.

Why This Matters
Scandium’s electron configuration helps us understand how it behaves in chemical reactions. It has one electron in the 3d orbital and two in the 4s orbital. This setup allows scandium to form a +3 oxidation state (Sc³⁺) in most of its compounds. This oxidation state is common for transition metals. It is also why scandium can make stable compounds and strong alloys.
Applications of Scandium
Scandium’s unique electron configuration makes it useful in several areas:
- Alloys: Scandium is added to aluminum to create strong, lightweight materials. These materials are used in airplanes, military vehicles, and sports equipment. The alloys offer strength, corrosion resistance, and good weldability, which are important for these applications.
- Lighting: Scandium is used in metal halide lamps. These lamps produce bright, white light that is similar to natural sunlight. This type of lighting is useful in film and television studios, theaters, and photography, where accurate color is important.
- Electronics: Scandium is also being explored for use in advanced electronics. It may be used in solid oxide fuel cells, which are an energy-efficient technology that could play a big role in the future of clean energy.
Chemical Reactivity of Scandium
Scandium is reactive, but less so than some other transition metals. In the air, it slowly forms a yellowish or pink oxide layer. This layer protects the metal from further oxidation. When exposed to water, scandium reacts slowly. It forms hydrogen gas and scandium hydroxide. These reactions show that scandium has medium reactivity compared to other transition metals.
Future Potential and Research
As technology advances, the demand for scandium is likely to grow. Researchers are looking for new ways to use scandium in 3D printing and renewable energy technologies. For example, scandium-aluminum alloys could become more common in industries that need lightweight, strong materials. In renewable energy, scandium might be used to make fuel cells more efficient.
Conclusion
The electron configuration of scandium, ArArAr 3d¹ 4s², is key to understanding its properties and uses. This configuration explains how scandium behaves in chemical reactions and why it can form valuable compounds and alloys. As scandium’s role in technology and industry grows, its electron configuration will remain important. Scandium’s contribution to modern technology, from creating stronger materials to advancing clean energy, is significant and continues to expand.
